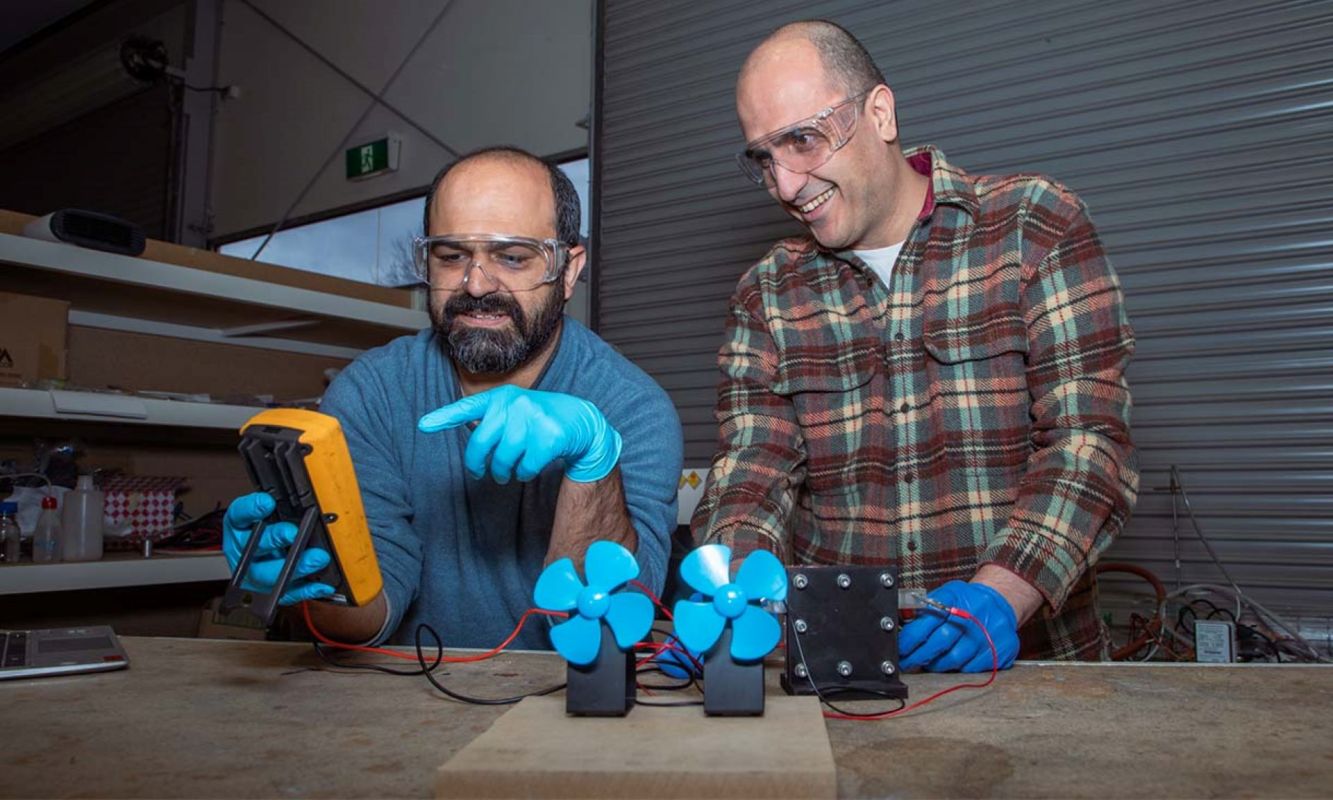
Researchers at the Royal Melbourne Institute of Technology have tripled the energy density of their experimental proton batteries, presenting an alternative to conventional lithium-ion batteries.
Lithium-ion batteries are everywhere. Chances are, they power your phone, laptop, appliances, and other electronics. Lithium-ion batteries have a high energy density, which means they can store a lot of energy in a relatively small space. Because of this, they are often used to power electric vehicles.
They don't come without a cost, though. Lithium and other rare earth metals necessary to make lithium-ion batteries are difficult to recycle. If they end up in landfills, they can release harmful toxins into the soil and nearby waterways. In addition, mining and processing lithium and the other metals that lithium-ion batteries require significant energy, contributing to air pollution that requires frequent use of the battery to offset. Finally, demand for EVs could soon outpace lithium supplies, threatening attempts to reduce reliance on fossil fuels.
The RMIT team's timing couldn't be better. Their new proton battery has an energy density of 245 watt hours per kilogram, nearly three times the energy density of the team's 2018 prototype. This energy density rivals that of conventional lithium-ion batteries, which typically have an energy density of around 260 Wh/kg.
Proton batteries use a carbon electrode and are charged by splitting water molecules.
"The main resource used in our proton battery is carbon, which is abundant, available in all countries, and cheap compared to the resources needed for other types of rechargeable battery such as lithium, cobalt, and vanadium," said lead researcher and RMIT professor John Andrews in a statement.
The battery is charged with a conventional power outlet. After that, electricity from the power supply splits water molecules, releasing protons that bond with the battery's carbon electrode. The battery can store these protons to be discharged and converted into power later.
"When discharging, protons are released from the carbon electrode and pass through a membrane to combine with oxygen to form water — this is the reaction that generates power," Andrews said.
Because they use abundant and readily available materials, these batteries are both cheap and more environmentally friendly than lithium-ion batteries. Aside from the power used to charge them, the production and usage of the batteries themselves generate almost no pollution. They don't require the mining and processing lithium-ion batteries do, and, unlike lithium-ion batteries, they're easily recyclable.
"As the world shifts to intermittent renewable energy to achieve net-zero greenhouse emissions, additional storage options that are efficient, cheap, safe, and have secure supply chains will be in high demand," Andrews said. "That's where this proton battery — which is a very equitable and safe technology — could have real value and why we are keen to continue developing it into a viable alternative."
The RMIT team will continue its partnership with automotive component supplier Eldor Corporation to further develop the technology.
Join our free newsletter for weekly updates on the coolest innovations improving our lives and saving our planet.